Ceramic vs. titanium implants: When to choose which?
Titanium implants have dominated dental implantology since its inception back in the 1960s. Subsequently, the products and solutions, the scientific and clinical research, and the development and performance of this treatment is overwhelmingly based on titanium implant systems.
For clinicians considering adding ceramic implants (specifically, zirconia) to their portfolio, an obvious question is when to choose ceramic vs. titanium implants, and why? Of course, every case and every patient is unique, but here are a number of factors you could consider if the indications make both ceramic and titanium implants a viable option.
- 1. Titanium implants – Benefits and challenges
- 2. Can patients be allergic to titanium dental implants?
- 3. Benefits of ceramic implants
- 4. Challenges with ceramic implants
- 5. Is zirconia really metal free?
- 6. Two-piece, screw-retained NobelPearl
- 7. Ceramic vs titanium implants – Conclusions
- 8. More to explore
Titanium implants – Benefits and challenges
- Scientific evidence: The very foundation of dental implantology was initiated by Prof P-I Brånemark over 65 years ago, after the discovery of natural bone attachment to titanium – osseointegration. Since this observation, a vast body of scientific evidence and decades of clinical cases have grown to underpin a solid basis of knowledge. Additionally, the wide ranges of applications and positive user experiences have served to create high levels of trust regarding the performance of many titanium implants.
- A wealth of indications: A vast number of products are available for many indications. For most indications a wide choice is available, all the way from single restorations for starters and general practitioners to the most complex cases of total rehabilitation, demonstrated by world-renowned experts in implantology. From a practical perspective, however, this vast choice may be overwhelming. This is one reason why adequate hands-on training is crucial for dental professionals when starting and progressing implant treatment.
- Strength: Titanium is highly resistant to external forces, and implant fractures are rare1 making it a solution in a vast array of cases where resistance of the material is crucial.2
One of the challenges with titanium implants is patient aversion to metallic medical devices being placed in their body, which can become displayed with mucosal recession and visibility of gray titanium. The emotional feel of a more ‘natural’ alternative may be of preference.
Can patients be allergic to titanium dental implants?
Allergy or hypersensitivity is sometimes posed as a drawback for titanium implants. However, it is important to note that allergic reactions appear to be very rare.3 In one study, just 0.6% of patients displayed reactions to titanium in an allergy test.4
If the patient is concerned that they may have an allergy to titanium, it is advisable to refer them to an allergist, and to act with caution in informing the patient that it is the definitive reason for placing a zirconia instead of titanium implant. If the patient has suffered implant failure and considers titanium allergy a possible reason, be aware that ‘rejection’ is most commonly associated with patient factors (such as smoking or hygiene), failure of the bioprocess of osseointegration or challenges with the surgical technique.5
Nonetheless, should there be concern over a potential patient reaction to titanium, a zirconia implant can provide a reassuring alternative.
Benefits of ceramic implants
- Esthetics: Perhaps the most distinctive yet simple difference between titanium and ceramic implants is the color. As a white material, the esthetic properties of ceramics are self-evident, especially in patients with a thin or delicate soft tissue biotype or in cases of soft tissue recession.6 Zirconia implants also lead to less mucosal discoloration than titanium.7 For patients who are very concerned about the potential visibility of titanium, especially in the anterior region, a ceramic implant may be a reassuring solution.
- Soft-tissue-friendly and less plaque: Looking deeper into the biology behind ceramic implants, several studies have shown that soft tissue attachment, low or weak inflammatory responses, and osseointegration are similar to those observed in titanium implants.8-12 Low affinity for attracting and retaining plaque has also been demonstrated13 as well as less bacterial adhesion than titanium.14,15

- A growing market: Ceramic implants are undoubtedly a niche market – estimated at around 1% – but it is growing.16 In terms of market share, it is expected to grow by up to 50% year-on-year over the next five years. Practices who want to stay ahead of the game and diversify their offerings may consider adding ceramic implants to their portfolio. For practices that emphasize esthetic solutions as a key differentiator of their services, offering the white color of ceramic implants could make them stand out from other local practices.
Challenges with ceramic implants
- Fewer clinical indications: At present, the clinical indications are limited compared to titanium implants. The current use of ceramic implants as a solution for tooth loss is mainly for single tooth replacement and bridge cases. There may be additional limitations in surgical and loading protocols.
- Predominantly one-piece cement-retained systems: Zirconia and titanium have very different material characteristics, so ceramic implants can’t simply replicate titanium implants. Until recently, zirconia has been mainly used as one-piece, cement-retained systems, which present several drawbacks in terms of the rigidity and stability of a cemented restoration.17 One-piece implants are less flexible than those with two parts secured by torqued screws. This limited flexibility also creates problems during placement of the implant, in the design of the restoration, and in the types of forces that may be exerted.18
- Cost of ceramic implants: The complex industrial process of manufacturing zirconia implants can impact the price. When selecting a ceramic implant, it is advisable to question the manufacturing method. With this brittle material, manufacturing flaws – even minute imperfections – in the production and surface treatment of a zirconia implant may compromise strength.19 Manufacturers should take great care with the materials for being clinically successful.
These drawbacks are a key reason for the design of NobelPearl™, summarized below, which is a two-piece, internally screw-retained system.
Is zirconia really metal free?
There is often some confusion amongst clinicians and patients regarding the difference between zirconium and zirconium dioxide (zirconia). Can you really call a zirconia implant “metal free” if it is derived from zirconium? Yes. ‘Zirconia’ is a ceramic material manufactured from zirconium dioxide. During the oxidation of zirconium, an irreversible chemical reaction occurs where electrons move from the zirconium to the oxygen molecules. As a result, ceramic zirconia has completely different material properties compared to the metal zirconium, including wear resistance, toughness and conductivity. This is why zirconia implants are described as ‘metal-free’.20-22
Two-piece, screw-retained NobelPearl
The ceramic implant offered by Nobel Biocare, NobelPearl, has been developed to provide a ceramic implant system overcoming some of the challenges mentioned above.* NobelPearl is a two-piece and screw-retained implant designed for restorative flexibility, and is available for a broad range of indications, from single to multiple unit.23 The screw itself is also 100% metal free, made of carbon-fiber-reinforced PEEK, a type of material that allows for a strong connection between the ceramic parts, but at the same time promotes uniform distribution of forces throughout the entire structure of the implant.24
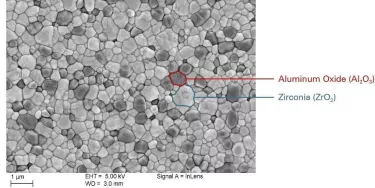
NobelPearl implants and abutments are milled from strong hot isostatic-pressed alumina-toughened zirconia (ATZ) blanks, and no sintering or finishing takes place after the final milling of the external and internal implant geometry, enabling a high level of dimensional precision and accuracy.25
The resulting material is biocompatible and radiopaque, with better hardness, bending strength, and toughness versus tetragonal zirconia polycrystals (TZP) used in other ceramic implants.12
Ceramic vs titanium implants – Conclusions
Ceramic implants are not a replacement for titanium implants, but an excellent alternative in a range of cases. Uniquely, they can meet the needs of a patient preference for 100% metal-free materials, with the esthetic reassurance of a white color. And, given this is still a niche market, it could potentially make a practice stand out in their local area.
From a clinical perspective, recent developments in zirconia solutions now mean that the restorative flexibility of a two-piece, screw-retained option is available with NobelPearl, along with proven osseointegration and soft tissue adhesion,26 generally lower plaque accumulation,27 and less bacterial adhesion than titanium implants.28,29
*See Instructions For Use for full prescribing information, including indications, contraindications, warnings and precautions.

We’re here to help
Unsure whether a ceramic or titanium implant is right for an upcoming case? Connect with your local sales rep to find answers to your questions.
More to explore
– Clinical case: Single-tooth restoration with the metal-free, two-piece ceramic implant system, NobelPearl
– More on the ceramic implant, NobelPearl: NobelPearl ceramic implant
References
1. Chrcanovic BR, Kisch J, Albrektsson T, Wennerberg A. Factors influencing the fracture of dental implants. Clin Implant Dent Relat Res. 2018 Feb;20(1):58-67. doi: 10.1111/cid.12572.
Read on PubMed
2. Osman R, Swain M. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015;8(3):932.
Read on PubMed
3. Albrektsson T, Chrcanovic B, Mölne J, Wennerberg A. Foreign body reactions, marginal bone loss and allergies in relation to titanium implants. Eur J Oral Implantol. 2018;11 Suppl 1:S37-S46.
Read on PubMed
4. Sicilia A, et al. Titanium allergy in dental implant patients: a clinical study on 1500 consecutive patients. Clin Oral Implants Res. 2008;19(8):823¬835.
Read on PubMed
5. Moy, P. K., D. Medina, V. Shetty and T. L. Aghaloo (2005). “Dental implant failure rates and associated risk factors.” Int J Oral Maxillofac Implants 20(4): 569-577.
Read on PubMed
6. Cosgarea R, Gasparik C, Dudea D, et al. Peri-implant soft tissue colour around titanium and zirconia abutments: a prospective randomized controlled clinical study. Clin Oral Implants Res 2015; 26(5):537–544.
Read on PubMed
7. Thoma DS, Ioannidis A, Cathomen E, et al. Discoloration of the peri-implant mucosa caused by zirconia and titanium implants. Int J Periodontics Restorative Dent 2016;36(1):39-45.
Read on PubMed
8. Cionca N, Hashim D, Cancela J, Giannopoulou C, Mombelli A. Pro-inflammatory cytokines at zirconia implants and teeth. A cross-sectional assessment. Clin Oral Investig 2016;20(8):2285-91.
Read on PubMed
9. Pieralli S, Kohal RJ, Jung RE, Vach K, Spies BC., Clinical Outcomes of Zirconia Dental Implants: A Systematic Review. J Dent Res. 2017 Jan;96(1):38-46.
Read on PubMed
10. Pieralli S, Kohal RJ, Lopez Hernandez E, Doerken S, Spies BC. Osseointegration of zirconia dental implants in animal investigations: A systematic review and metaanalysis. Dental Materials 2018;34(2):171-82.
Read on PubMed
11. Chappuis V, Cavusoglu Y, Gruber R, et al. Osseointegration of Zirconia in the Presence of Multinucleated Giant Cells. Clin Implant Dent Relat Res 2016;18(4):68698.
Read on PubMed
12. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000. 2017;73(1):241-258.
Read on PubMed
13. Scarano A et al., Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study., J Periodontol. 2004 Feb; 75(2):292–296.
Read on PubMed
14. Rimondini L, Cerroni L, Carrassi A, et al. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants 2002; 17:793–798.
Read on PubMed
15. Nobel Biocare market analysis. iData report, 2017.
16. Kohal RJ, Spies BC, Bauer A, Butz F. One-piece zirconia oral implants for single-tooth replacement: Three-year results from a long-term prospective cohort study. J ClinPeriodontol 2018;45(1):114-24.
Read on PubMed
17. Osman R, Swain M. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015;8(3):932.
Read on PubMed
18. Osman R.B., Ma S., Duncan W., de Silva R.K., Siddiqi A., Swain M.V. Fractured Zirconia implants and related implant designs: Scanning electron microscopy analysis. Clin. Oral Implants Res. 2013;24:592–597. doi: 10.1111/j.1600-0501.2011.02411.x.
Read on PubMed
19. Edelhoff D, Schweiger J, Prandtner O, Stimmelmayr M, Güth JF. Metal-free implant-supported single-tooth restorations. Part II: Hybrid abutment crowns and material selection. Quintessence Int. 2019;50(4):260-269
Read on PubMed
20. Jianmin Han, Jing Zhao & Zhijian Shen. Zirconia ceramics in metal-free implant dentistry, Advances in Applied Ceramics, 2017, 116:3, 138-150, DOI: 10.1080/17436753.2016.1264537
Read online
21. Bollen CM (2017) Zirconia: The Material of Choice in Implant Dentistry? An Update. J Dent Health Oral Disord Ther 6(6): 00219. DOI: 10.15406/jdhodt.2017.06.00219.
Read on ResearchGate
22. Nobel Biocare. Data on file.
23. Tartsch J. Keramikimplantate – Exoten oder sinnvolle Erweiterung des Behandlungsspektrums? ZMK 2018;11(34):750-760.
24. Nobel Biocare. Data on file.
25. Spies BC, Sauter C, Wolkewitz M, Kohal RJ. Alumina reinforced zirconia implants: effects of cyclic loading and abutment modification on fracture resistance. Dent Mater 2015;31(3):262-272.
Read on PubMed
26. Scarano A et al., Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study., J Periodontol. 2004 Feb; 75(2):292–296.
Read on PubMed
27. Rimondini L, Cerroni L, Carrassi A, et al. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants 2002; 17:793–798.
Read on PubMed
28. Nobel Biocare market analysis. iData report, 2017.