
creos™ xenogain
Three methods of application to meet all your bone grafting needs.
Regenerating bone for 15 years
Three different methods of application
The portfolio offers a selection of xenogenic bone substitute for guided bone regeneration (GBR) and guided tissue regeneration (GTR).
creos xenogain is provided sterile and is available in a vial, in a syringe, or a bowl for fast and easy application.
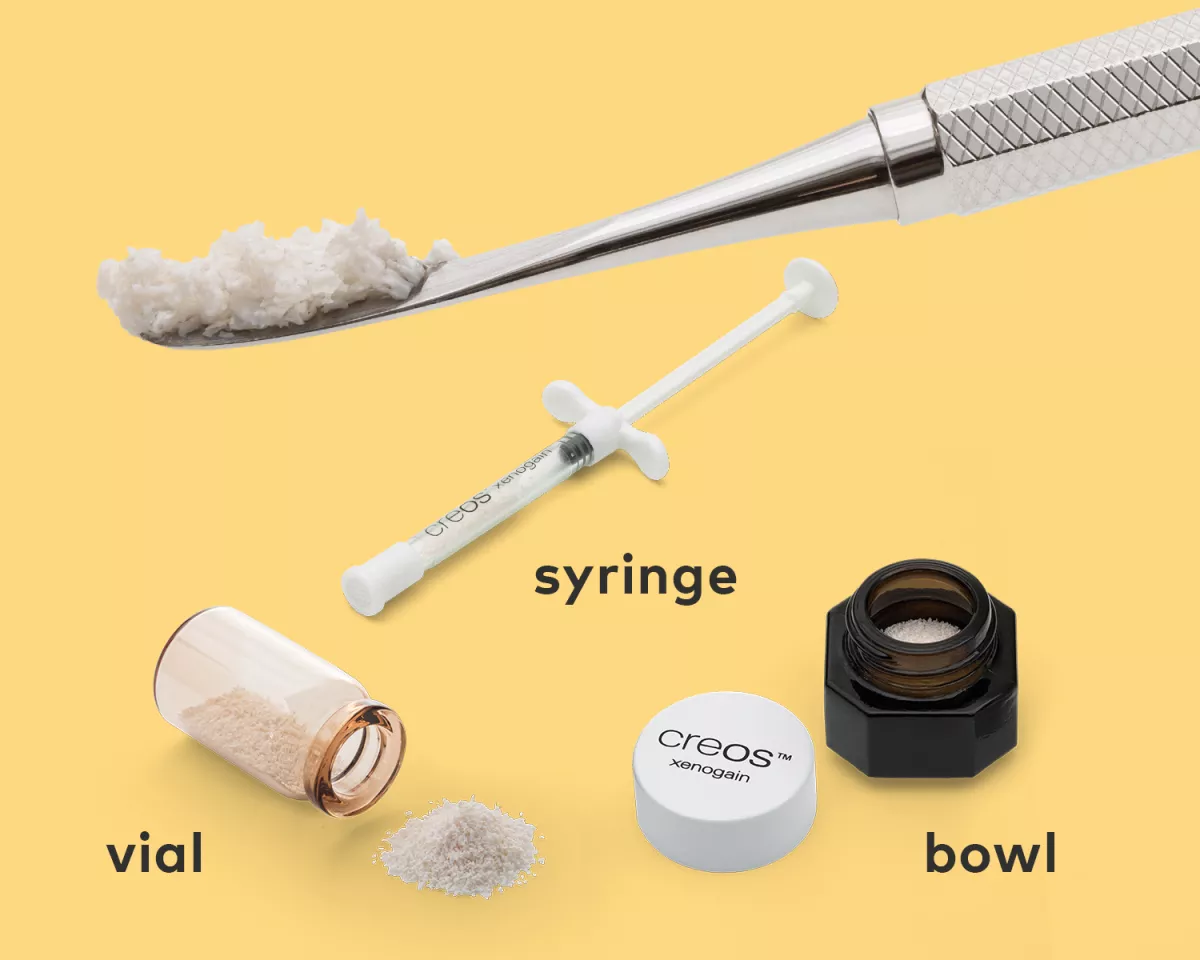
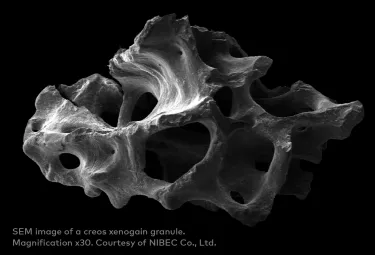
creos xenogain particles are made of deproteinized bovine bone mineral matrix with preserved micro- and macrostructures.1
Why creos™ xenogain?
"I appreciated its handling properties and I see its high hydrophilicity as a biological advantage in sinus grafting and peri-implant defect regeneration.”
Scaffold for successful regeneration
Preserved natural features of bone through optimized manufacturing process.4
Chemical composition
With a calcium phosphate ratio that reflects the composition in human bone and a structure with low crystallinity.
The body accepts creos xenogain as a suitable framework for bone formation.1
Solid foundation for implant placement
The graft integrates with the newly formed bone, building a basis for successful implant placement.2
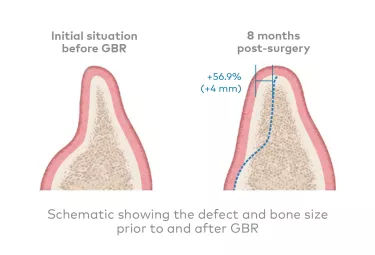
In a multicenter clinical study involving 46 patients, bone increase after 8 months was 4.0 mm (+56.9 % gain) and 4.7 mm (51.0 % gain) at 1 and 3 mm from the top of the crest, respectively.6
GBR led to robust bone regeneration during the 8 months of healing, enabling successful placement of 91 implants in 43 patients, with an average insertion torque of 37.8 ± 5.1 Ncm.6
-
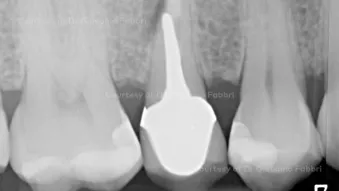 Immediate placement and provisionalization with fully digital workflow
Immediate placement and provisionalization with fully digital workflowClinician: Dr. Giacomo Fabbri
-
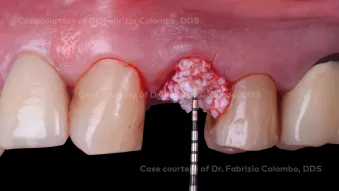 Immediate loading of post extractive single tooth in esthetic zone with simultaneous GBR
Immediate loading of post extractive single tooth in esthetic zone with simultaneous GBRClinician: Dr. Fabrizio Colombo, DDS
-
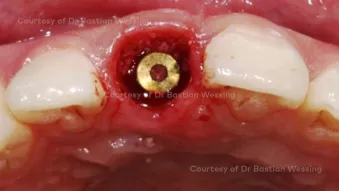 Buccal bone augmentation during immediate implant placement
Buccal bone augmentation during immediate implant placementClinician: Dr. Bastian Wessing
Questions about creos xenogain?
If you would like additional information, more details, or have specific questions about creos xenogain, click the link below.

Find a course on hard and soft tissue management
References
- Nobel Biocare, data on file.
- Park JB, et al. Maxillary sinus floor augmentation using deproteinized bovine bone-derived bone graft material (OCS-B). Clinical and histologic findings in humans. The Journal of the Korean Dental Association. 2007;45(8):491–99.
- Park HN, Han, SH, Kim KW, et al. A study on the safety and efficacy of bovine bone-derived bone graft material (OCS-B). J Korean Acad Periodontol. 2005 Jun;35(2):335–43.
- Rhee S-H, Park HN, Seol Y-J et al. Effect of heat-treatment temperature on the osteoconductivity of the apatite derived from bovine bone. 2006 Key Engineering Materials 309-311:41-44
- Shin S-Y, et al. Long-term results of new deproteinized bovine bone material in a maxillary sinus graft procedure. J Periodontal Implant Sci.2014;44;259-64.
Read on Pubmed - Aleksic Z, Milikovic I, Laziv Z, et al. A multicenter clinical investigation demonstrates bone regeneration in severe horizontal defects in the posterior mandible using creos™ xenoprotect: Interim results. J Clin Periodontol 2018;45(S19):306.
Read poster - Kim Y-T, et.al. Periodontal Repair on Intrabony Defects treated with Anorganic Bovine-derived Xenograft.J Korean Acad Periodontol. 2007;37(3):489–96.



